Optimization of a Ferrate Process for Degradation of a Refractory Compound
Abstract
Ferrate(VI) was synthesized by wet oxidation method and has been applied to the degradation of perchloroethylene(PCE) in this study. In order to provide information about degradation efficiency of PCE by ferrate(VI), several parameters such as pH, ferrate(VI) dosage, and temperature were investigated. The degradation of PCE by ferrate(IV) follows the second order reaction kinetics. The optimal reaction rate constant of 315.19 M-1s-1 was obtained in neutral pH condition, and the activation energy of 35.7 kJ/mole was for PCE. PCE degradation pathways were proposed based on reaction intermediates identified by GC/MS.
Keywords:
Wet Oxidation Method, Oxidant, Refractory Compound, Degradation Pathways1. Introduction
Iron(Fe) commonly exists in the +2(ferrous) and +3(ferric) oxidation states. Under strong oxidizing environments, iron moves to higher oxidation states such as +6(ferrate) oxidation state. Hexavalent iron or ferrate(VI) is a strong oxidizing chemical throughout the entire pH range, from 2.2V in acid and 0.7V in base, as listed in the following equations:
FeO42- + 8H+ + 3e- ↔ Fe3+ + 4H2OEo = 2.2 V (in acidic condition)
FeO42- + 4H2O + 3e- ↔ Fe(OH)3 + 5OH-
Eo = 0.7V (in basic condition)
Fe(VI) oxidation with a large number of organic compounds have been studied and it has been shown that Fe(VI) is a selective oxidant1-6). The reduction of Fe(VI) results in a relatively non-toxic by-product iron(III), which suggests that ferrate(VI) is an environmentally friendly oxidant. Due to many beneficial properties of ferrate(VI) for water and waste water treatments, synthesis of ferrate(VI) became very important in order to provide new promising materials for water and wastewater treatment. In 1897, Moeser was the first to write a detailed review of ferrate and their chemistry describing three methods of preparation of potassium ferrate, namely: Dry method-heating to red various potassium and iron containing minerals. Dry reactions imply detonation and elevated temperatures, which are considered dangerous and too difficult to implement; Electrochemical method-electrolyzing a potash solution with an iron anode. This method remains another access to ferrates that has retained only marginal interest7). Ferrate(VI) was prepared in an electrochemical way for the first time as early as 1841. Since then, it has benefited from recent improvements though, such as superposition of an alternative current to the direct current for electrolysis,8,9) and Wet method-oxidizing a basic solution of a Fe(III) salt by a hypochlorite or hypobromide.10-14) The organic compounds and pathogens can be oxidized/ disinfected by ferrate(VI) effectively without disinfection by-products (DBPs).5)
PCE is a representative volatile organic compound that the International Agency for Research on Cancer considers to be a likely carcinogen. Due to being non-inflammable, chemically stable, and an excellent washing solvent15), PCE is frequently used in commercial processes such as machine manufacturing, metal degreasing, and dry cleaning; and as a result, many cases of PCE-contaminated groundwater and soil have been reported.16) Research and experiment in this area became very interesting due to the properties and abilities of ferrate(VI) such a promising technology in environmental technology. This study would like to provide the information about optimum condition for making useable ferrate(VI) in the solution by wet oxidation method with high concentration and application to degrade PCE.
2. Experimental setup and method
2.1 Materials
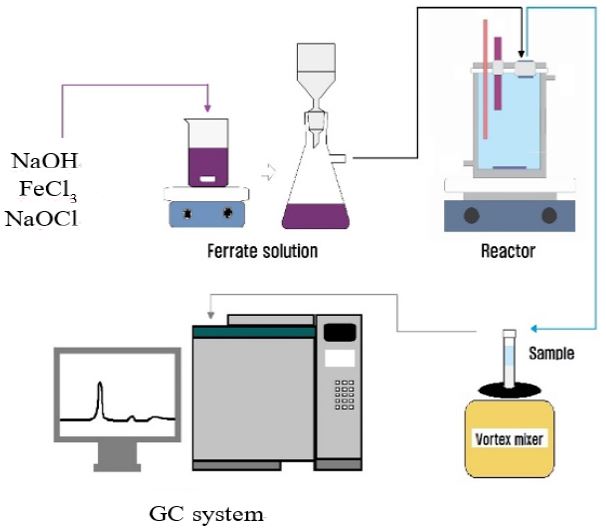
In this study, the chemicals used were reagent grade or higher purity to synthesis ferrate(VI) using wet oxidation method reported by Schreyer. Iron source FeCl3.6H2O, as oxidizing agent sodium hypochlorite and sodium hydroxide were used in this experiment. Agilent GC-ECD(gas-chromatography) was used to measure PCE concentration in degradation by ferrate experiment. The intermediate product analysis was performed using a GC/MS-Mass Spectrometer Systems(GCMS-QP2010Plus, Shimadzu, Japan) was used. Fig. 1 shows the schematic diagram of experimental set-up.
2.2 Experimental Procedure
Ferrate(VI) generated from synthesis was analyzed using spectrophotometer DR 5000, HACH Company at 505 nm in buffer solution to measure concentration of ferrate(VI) produced. After the maximum concentration of ferrate(VI) was obtained, ferrates(VI) were used in degradation of PCE.
PCE was used as the refractory organic compound due to its properties that has been reported to be assessed as carcinogenic to animals and affect liver damage and kidney failure to humans.15) The experiments were performed in the batch mode. The glass reactor was in closed zero-head space status and its lid was sealed with Teflon covered rubber stoppers. In this experiment pH, ferrate dosage and temperature have been studied at various conditions to determine the degradation process of PCE using ferrate from the synthesis. In order to measure the PCE concentration after degradation process Agilent GC-ECD was used.
This experiment was carried out to identify the intermediate products from degradation of PCE by ferrate(VI). Sample from the degradation reactor were analyzed using GC/MS Mass System (GCMS-QP2010Plus, Shimadzu, Japan) and AT-1 capillary column(60 m×0.32 mm I.D×1.0 micrometer film thickness). To prevent further degradation by ferrate, sample taken from reactor were quenched using NaOH.
3. Experimental results and discussion
Wet oxidation method uses oxidant to transform iron (Fe) from low oxidation states to the highest oxidation states. This method was the easiest method among three methods for ferrate(VI) synthesis. In this study sodium hypochlorite and sodium hydroxide were used as the oxidation agent.13) Oxidizing process is the key of making iron(Fe) in the high oxidation states. The aim of the study was to provide high concentration of ferrate(VI) by wet oxidation method. 60 ml of sodium hypochlorite(NaClO) and 31 gram sodium hydroxide (NaOH) were reacted in the beaker then 4 grams of iron source(FeCl3.6H2O) were added to generate ferrate(VI). An optimum time was found out at 12 minutes using spectrophotometer DR 5000, HACH company with wavelength 505 nm.
In the application experiment of PCE degradation, ferrates(VI) generated from the synthesis were used to degrade PCE in closed zero-head space glass reactor. To obtain the greatest amount of PCE degraded from the reactor, several parameters were investigated such as pH varying from 3 to 11, ferrate(VI) dosage from 14 ppm to 56 ppm and temperature variation varying from 10℃ to 45℃. All experiments were performed to determine the highest degradation efficiency of ferrate(VI) in the degradation of PCE.
The pH effect on the degradation of PCE were studied with C0 of PCE 1 ppm while ferrate(VI) concentration was 0.5 mL(±28 ppm) in the temperature of 25℃. The best degradation efficiency was obtained in pH 5.5-7.5 with 60% of PCE removal. The pH effect on PCE degradation is depicted in Fig. 2. Ferrate(VI) was a very strong oxidizing agent throughout almost whole pH range with a redox potential varying from +0.7V in basic solution to +2.2V in acidic solution. But ferrate(VI) ion reduced rapidly to oxygen and Fe(III) in strong acids, while the oxygen ligands of ferrate(VI) exchanged very slowly with water at pH 10. In this experiment ferrate(VI) in the form of FeO42- decomposed very fast with decreasing of pH. From the experiment in acidic condition ferrate(VI) decomposed 34-40% and in neutral 25-29% decomposed within beginning of 30 seconds. According to the previous studies on ferrate(VI) reaction with organic compound, second order reaction rate was obtained17). The second order reaction rate law can be described by equation (1).
| (1) |
Eq (1) is rearranged and d[PCE]/dt is integrated to become.
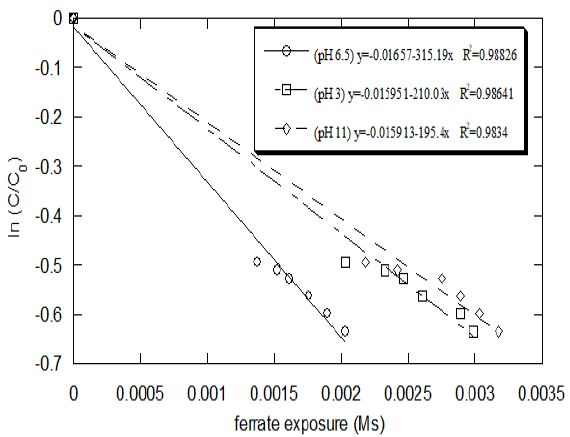
Where is the ferrate concentration exposure, and the kapp is the apparent second order reaction rate constant.17-19) From this experiment the degradation rate can be showed by kapp value shown in Fig. 2, Where the highest degradation efficiency obtained in pH neutral condition with kapp of 315.19 M-1s-1.
The value of kapp could be obtained by plotting the natural logarithm of PCE concentration(ln C/Co) vs. the ferrate exposure. From the ferrate exposure and PCE degradation, the rate of reaction could be determined. This approaching method was used to measure all kapp in different variation of parameters from PCE degradation experiment including temperature variations. From this experiment the highest degradation efficiency obtained in pH neutral (6.5) with 315.19 M-1s-1, while the kapp value of 210.3 M-1s-1 and 195.4 M-1s-1 were obtained in pH 3 and pH 11, respectively. Even though pH neutral showed the highest degradation efficiency. Acid condition was used for other parameter experiments because controlling to keep the pH neutral condition was very difficult.
Ferrate(VI) was added with different concentrations to determine the effect of ferrate(VI) concentration or dosage on degradation of PCE. The experiments used ferrate(VI) dosages in this range from 14 to 56 ppm. The results showed linear trends as increasing ferrate(VI) dosage. Increasing degradation efficiency was because increasing of ferrate(VI) dosage, which increases the amount of ferrate available for degrading PCE. The ferrate(VI) dosage effect on the degradation of PCE is shown in Fig. 3.
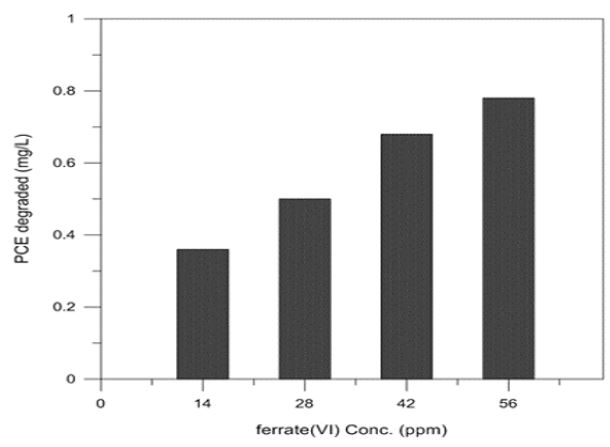
PCE degraded(mg/L) in ferrate dosage variations at pH 3 with 1 ppm initial concentration, temperature 25oC, contact time 10 minutes.
The degradation of refractory organic compounds was strongly depended on the dosage of ferrate(VI) added to the reactor.20) Fig. 3 shows the proportional relationship between ferrate(VI) dosage and degradation of PCE. A linear trend has also been observed in the degradation of PCE with increasing of the ferrate(VI) dosage.21)
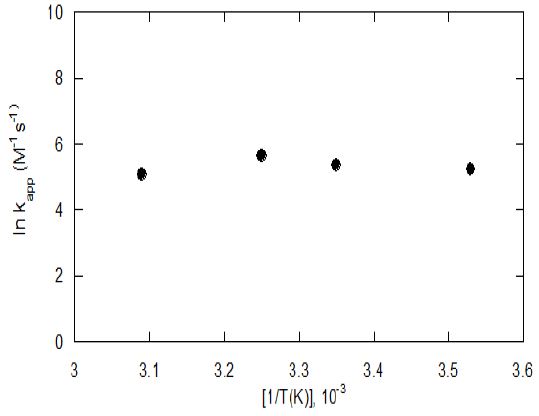
The experiments were conducted to determine the temperature effect on the degradation efficiency of PCE by ferrate(VI). The experiments were carried out following the previous temperature conditions to compare and showed similar results.22) The results showed that optimum temperature of PCE degradation using ferrate(VI) was 25℃. In the experiment a second order reaction was identified in the PCE degradation by ferrate(VI). The temperature seems to be a very important parameter, because the temperature effect on ferrate(VI) stability has been reported by many researchers through their previous studies. Increase in temperature caused an improvement of the homogeneous ferrate(VI) decomposition kinetics.21-24) The activation energy which was measured by Arrhenius equation from experiment data is 35.7 KJ/mole; where ea is activation energy; R is constant value(8.314 J K-1 mole-1); and T is temperature (K).
| (2) |
Fig. 4 shows the values of kapp in temperature range from 10℃ to 45℃.
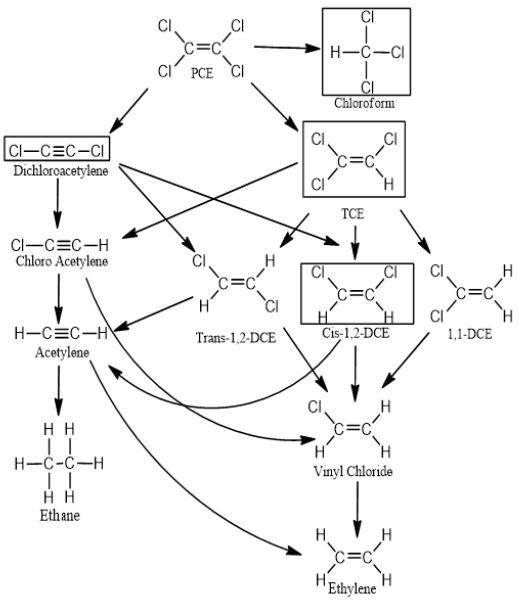
This experiment was performed to identify the intermediate products during the degradation reaction of PCE by ferrate(VI). Samples from the degradation reactor were analyzed using GC/ MS. The degradation mechanisms between ferrate(VI) and target compounds have been divided into three categories. Generally, ferrate(VI) was known to have target compounds to be oxidized either by supplying oxygen or by taking away electrons or hydrogen atoms from target compounds. The reaction was brought about by providing oxygen, which was provided mainly from ferrate(VI).25,26) Fig. 5 depicts the possible intermediates which have been detected similarly 22) and the reaction pathways involving oxygen. The first stage was the oxygen transferring and proton addition which produces trichloroethylene (TCE), dichloroacetylene and chloroform. Trichloroethylene could be converted to 1,2-DCE by the second oxygen transferring. After second oxygen transferring, 1,2-DCE(dichloroethene) could be converted to vinyl chloride by third oxygen transferring. Vinyl chloride could be converted to ethylene. Further decomposition would generate ethane.27,28) TCE degradation pathways propose that the ethane would convert to glyoxylic acid and glycolic acid. Then it was proposed that further decomposition of the reaction intermediates led to the end products like carbon dioxide.29) Dichloroacetylene could be changed to chloroacetylene by second oxygen transfer.
4. Conclusions
Ferrate(VI) is a powerful oxidizing agent and its oxidizing power can be used in advanced oxidizing process. In order to investigate degradation efficiency of PCE by ferrate(VI), several parameters such as pH, ferrate(VI) dosage, and temperature were studied. The degradation of PCE by ferrate(IV) follows the second order reaction kinetics. The optimal reaction rate constant was obtained in neutral pH condition, and the activation energy of 35.7 kJ/mole was for the degradation of PCE by ferrate(IV). PCE degradation pathways were proposed based on reaction intermediates identified by GC/MS. The decomposition of PCE by ferrate(IV) produced 1,2-DCE(dichloroethene), TCE, dichloroacetylene, dichloroethylene, and chloroform as the reaction intermediates.
References
- R. J. Audette, J. W. Quail, and P. J. Smith, (1971), "Ferrate (VI) ion, a novel oxidizing agent", Tetrahedron Lett, 3, p279-281.
-
D. H. Williams, and J. T. Riley, (1974), "Preparation and Alcohol Oxidation Studies of the Ferrate (VI) ion FeO42-", Inorganica Chim. Acta, 8, p177-183.
[https://doi.org/10.1016/s0020-1693(00)92612-4]

- Y. Tsuda, and S. Nakajima, (1978), "Potassium Ferrate, a new selective oxidizing agent", Chem. Lett, p1397-1398.
- Carr, (1985), "Properties of Ferrate(VI) in aqueous solution: an alternate oxidant in waste water treatment", In Jolley, R. L. (Ed), Proceedings of Conference on Water Chlorination Chem, Environment Impact Health Eff Lewis Chelsew, p1285-1298.
- L. Delaude, and P. Laszlo, (1996), "A novel oxidizing reagent based on Potassium Ferrate (VI)", J. Org. Chem, 61, p6360-6370.
-
C. Li, X. Z. Li, and N. Graham, (2005), "A Study of the preparation and reactivity of potassium ferrate", Chemosphere, 61, p537-543.
[https://doi.org/10.1016/j.chemosphere.2005.02.027]

- L. Moeser, (1897), J. Prakt. Chem, 56, p425.
- K. Bouzek, and I. Rousar, (1993), Elektrochim, Acta, 38, p1717-1720.
- B. H. Bielski, (1991), "Studies of hypervalent ion", Free. Rad. Res. Commns, 12(13), p469-477.
-
M. Thompson, L. T. Ockerman, and J. M. Schreyer, (1951), "Preparation and Purification of Potassium ferrate (VI)", J. Am. Chem. Soc., 73, p1379-1381.
[https://doi.org/10.1021/ja01147a536]

- J. M. Schreyer, W. Thompson, and L. T. Ockerman, (1953), "Potassium Ferrate (VI).", Inorg. Synthesis, 4, p164-169.
-
D. A. White, and G. S. Franklin, (1998), "A Preliminary Investigation into the Use of Sodium Ferrate in Water Treatment", Environ. Technol., 19(11), p1157-1160.
[https://doi.org/10.1080/09593331908616776]

- J. Chengchun, L. chen, and W. Shichao, (2008), "Preparation of potassium ferrate by wet oxidation method using waste alkali: Purification and reuse of waste alkali", ACS Symposium Series, 985, p94-101.
- H. Hrostowski, and A. Scott, (1960), J. Chem. Phys., 18, p105.
- W. Martha, B. Susan, Y.S. Lorraine, and N. F. Margaret, (1983), The Merck Iindex, 10th ed, Merck & Co., Rahway, p1315.
- K. Hirata, (1996), Counterplan of Soil and Groundwater Pollution, first ed, Nihon kankyo sokuteibunsekikyokai, p3-14.
- Y. Bing, Y. Guang-Guo, Z. Li-Juan, Z. Li-Jun, L. Shan, and0 F. Yi-Xiang, (2011), Kinetics modeling and reaction mechanism of ferrate(VI) oxidation of benzotriazoles, Wat. Res., 45, p2261-2269.
- V. K. Sharma, (2013), Ferrate(VI) and ferrate(V) oxidation of organic compounds: kinetics and mechanism, Coordi. Chem. Rev., 257(2), p495-510.
-
B. Yang, and G. G Ying, (2011), Oxidation of triclosan by ferrate: Reaction kinetics, products identification and toxicity evaluation, J. of Haz. Mat., 186, p227-235.
[https://doi.org/10.1016/j.jhazmat.2010.10.106]

- M. Yu, G. Park, and H. O. Kim, (2008), Oxidation of Nonylphenol Using Ferrate, (2008) ACS, 24, p389-403.
-
W. F. Wagner, J. R. Gump, and E. N. Hart, (1952), Factors affecting stability of aqueous potassium ferrate(VI) solutions, Anal. Chem., 24, p1497-1498.
[https://doi.org/10.1021/ac60069a037]

-
Kim, I. K., (2015), Degradation of perchloroethylene by ferrate(VI), Korean Soc. Wat. Wastewat., 29(1), p39-46.
[https://doi.org/10.11001/jksww.2015.29.1.039]

- T. Svanks, (1976), Oxidation of ammonia in water by and ferrates(VI) and (IV), Wat. Resources Center, Engineering Experiment Station, The ohio state university, Columbus.
- Z. Macova, K. Bouzek, J. Hives, V. K. Sharma, R.J. Terryn, and J. C. Baum, (2009), Research progress in the electrochemical synthesis of ferrate(VI), Electrochimica Acta., 54, p2673-2683.
-
V. K. Sharma, (2003), "Potassium Ferrate (VI): An environmentally friendly oxidant", Adv. Environ. Res., 6(2), p143-156.
[https://doi.org/10.1016/s1093-0191(01)00119-8]

-
J. Q. Jiang, and B. Lloyd, (2002), Progress in the development and use of ferrate(VI) salt as an oxidant and coagulant for water and wastewater treatment, Wat. Res., 36, p1397-1408.
[https://doi.org/10.1016/s0043-1354(01)00358-x]

-
H. Y. Jeong, H. Kim, and K.F. Hayes, (2007), Reductive dechlorination pathways of tetrachloroethylene and trichloroethylene and subsequent transformation of their dechlorination products by mackinawite(FeS) in the presence of metals, Environ. Sci. Technol., 41, p7736-7743.
[https://doi.org/10.1021/es0708518]

-
W. A. Arnold, and A. L. Roberts, (2000), Pathways and kinetics of chlorinated ethylene and chlorinated acetylene reaction with Fe(0) particles, Environ. Sci. Technol., 34, p1794-1805.
[https://doi.org/10.1021/es990884q]

-
M. Kang, J. H. Lee, S. H. Lee, C. H. Chung, K. J. Yoon, K. Ogino, S. Miyata, and S. J. Choung, (2003), Preparation of TiO2 film by the MOCVD method and analysis for decomposition of trichloroethylene using in situ FT-IR spectroscopy, J. Molecular Catalysis A: Chem., 193, p273-783.
[https://doi.org/10.1016/s1381-1169(02)00474-0]